Standard Vs Non Standard Cell Potential . this equation describes how the potential of a redox system (such as a galvanic cell) varies from its standard state value, specifically,. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. cell potential if a cell at standard conditions can be obtained by the equation: The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. standard cell potential.
from www.chegg.com
cell potential if a cell at standard conditions can be obtained by the equation: this equation describes how the potential of a redox system (such as a galvanic cell) varies from its standard state value, specifically,. standard cell potential. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell.
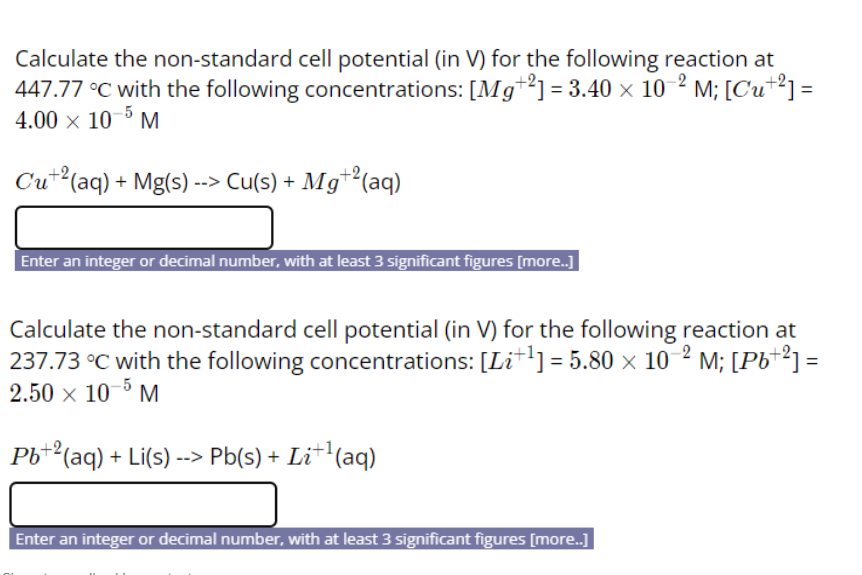
Solved Calculate the nonstandard cell potential (in V) for
Standard Vs Non Standard Cell Potential 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. cell potential if a cell at standard conditions can be obtained by the equation: standard cell potential. this equation describes how the potential of a redox system (such as a galvanic cell) varies from its standard state value, specifically,.
From app.pandai.org
Standard Electrode Potential Standard Vs Non Standard Cell Potential The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. standard cell potential. this equation describes how the potential of a redox system (such as a galvanic cell) varies from. Standard Vs Non Standard Cell Potential.
From www.chegg.com
Solved The Nernst Equation enables the determination of cell Standard Vs Non Standard Cell Potential The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. this equation describes how the potential of a redox system (such as a galvanic cell) varies from its standard state value, specifically,. cell potential if a cell at standard conditions can be obtained by the equation: . Standard Vs Non Standard Cell Potential.
From slideplayer.com
Voltaic Cells. ppt download Standard Vs Non Standard Cell Potential The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. cell potential if a cell at standard conditions can be obtained by the equation: 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. standard cell potential. this equation. Standard Vs Non Standard Cell Potential.
From slideplayer.com
Equilibrium Electrochemistry ppt download Standard Vs Non Standard Cell Potential 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. standard cell potential. this equation describes how the potential of a redox system (such as a galvanic cell) varies from. Standard Vs Non Standard Cell Potential.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps Standard Vs Non Standard Cell Potential The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. standard cell potential. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. cell potential if a cell at standard conditions can be obtained by the equation: this equation. Standard Vs Non Standard Cell Potential.
From www.chegg.com
Solved Calculate the nonstandard cell potential (in V) for Standard Vs Non Standard Cell Potential cell potential if a cell at standard conditions can be obtained by the equation: standard cell potential. this equation describes how the potential of a redox system (such as a galvanic cell) varies from its standard state value, specifically,. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The. Standard Vs Non Standard Cell Potential.
From www.youtube.com
Calculate the cell potential at standard and then at nonstandard Standard Vs Non Standard Cell Potential cell potential if a cell at standard conditions can be obtained by the equation: this equation describes how the potential of a redox system (such as a galvanic cell) varies from its standard state value, specifically,. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. . Standard Vs Non Standard Cell Potential.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Standard Vs Non Standard Cell Potential this equation describes how the potential of a redox system (such as a galvanic cell) varies from its standard state value, specifically,. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. cell potential if a cell at standard conditions can be obtained by the equation: . Standard Vs Non Standard Cell Potential.
From scienceinfo.com
Cell Potential & Standard Cell Potential Standard Vs Non Standard Cell Potential cell potential if a cell at standard conditions can be obtained by the equation: The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. standard cell potential. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. this equation. Standard Vs Non Standard Cell Potential.
From www.slideserve.com
PPT Electrochemical Potential Non Standard Conditions Variations in Standard Vs Non Standard Cell Potential cell potential if a cell at standard conditions can be obtained by the equation: The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. standard cell potential. this equation. Standard Vs Non Standard Cell Potential.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 Standard Vs Non Standard Cell Potential 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. standard cell potential. this equation describes how the potential of a redox system (such as a galvanic cell) varies from its standard state value, specifically,. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates. Standard Vs Non Standard Cell Potential.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Vs Non Standard Cell Potential standard cell potential. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. cell potential if a cell at standard conditions can be obtained by the equation: this equation describes how the potential of a redox system (such as a galvanic cell) varies from its standard. Standard Vs Non Standard Cell Potential.
From www.chegg.com
Solved HW29.1. NonStandard Cell Potentials Determine the Standard Vs Non Standard Cell Potential this equation describes how the potential of a redox system (such as a galvanic cell) varies from its standard state value, specifically,. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the. Standard Vs Non Standard Cell Potential.
From www.youtube.com
Cell Voltages NonStandard Conditions YouTube Standard Vs Non Standard Cell Potential this equation describes how the potential of a redox system (such as a galvanic cell) varies from its standard state value, specifically,. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. standard cell potential. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates. Standard Vs Non Standard Cell Potential.
From www.chegg.com
Solved Calculate the nonstandard cell potential (in V) for Standard Vs Non Standard Cell Potential 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. cell potential if a cell at standard conditions can be obtained by the equation: The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. standard cell potential. this equation. Standard Vs Non Standard Cell Potential.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID321506 Standard Vs Non Standard Cell Potential 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. standard cell potential. this equation describes how the potential of a redox system (such as a galvanic cell) varies from its standard state value, specifically,. cell potential if a cell at standard conditions can be obtained by the equation: The. Standard Vs Non Standard Cell Potential.
From www.geeksforgeeks.org
What is Cell Potential Definition, Formula, and Examples Standard Vs Non Standard Cell Potential cell potential if a cell at standard conditions can be obtained by the equation: standard cell potential. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. this equation. Standard Vs Non Standard Cell Potential.
From www.slideserve.com
PPT Electrochemical Equilibria PowerPoint Presentation, free download Standard Vs Non Standard Cell Potential standard cell potential. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. this equation describes how the potential of a redox system (such as a galvanic cell) varies from. Standard Vs Non Standard Cell Potential.